Repair. Regenerate. Restore.
Despite great progress of mechanical repairs in soft tissue procedures, healing rates remain variable driving the need for biologic enhancement. The historical challenge of creating biologic augmentation has been balancing biological enhancement and structural performance. Leveraging additive manufacturing platforms, such as electrospinning and microfluidic extrusion, has allowed Embody to address prior limitations. Embody’s collagen-based biologic products are designed to optimize both structure and chemistry creating an ideal microenvironment for healing.
Technology Platforms
BIOSPIN™
Microfibrous biofabrication technology for soft tissue augmentation with biomechanical performance
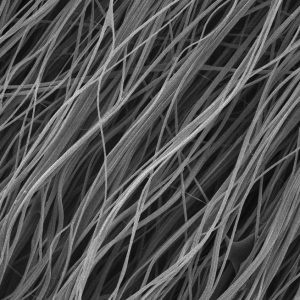
BIOSPIN, a hybrid electrospun / pneumatospun technology for highly organized, highly porous collagen-based microfibrous devices designed for soft tissue augmentation. The BIOSPIN platform technology is used in our TAPESTRY® Biointegrative Implant for tendon and ligament augmentation.
Our PRODUCTS
TAPESTRY Biointegrative Implant is a bioengineered implant with a highly aligned & highly porous architecture combined with type I bovine collagen chemistry specifically designed for tendon and ligament repair.
TAPESTRY RC Arthroscopic Delivery and Fixation System is the first arthroscopic implant system for rotator cuff that combines a biointegrative collagen-based implant with fully bioabsorbable fixation and streamlined arthroscopic delivery for partial to full thickness rotator cuff tears.
Embody Patents
Embody patent information is provided to satisfy the notice requirement for the virtual patent marking provision of the America Invents Act. The following list may not be all inclusive.
11,338,056
Microfluidic Extrusion
US 11,338,057
Microfluidic Extrusion
US D946,768
Insertion Device (Achilles)
US D946,767
Design Patent Insertion Device (Achilles)
US 11,259,939
Graft Inserter
US 11,213,610
Biopolymer Scaffolds and Methods for their Production
US 11,020,509
Microfluidic Extrusion of Collagen Fibers
US 11,116,870
Biopolymer Compositions, Scaffolds and Devices
US 10,966,815
Enclosure Device for an Implantable Repair Device
US 10,835,639
Bioploymer Compositions, Scaffolds and Devices
US 10,653,817
Method for Producing an Implantable Ligament and Tendon Repair Device
US 10,617,787
Biopolymer Compositions, Scaffolds and Devices




